Learn how Oncology Consultants ensured timely documentation and sign-off of study patient data by leveraging research documentation in OncoEMR®.
KPI Improvements with Flatiron
-
More timely data documentation and sign-off
- 5 hours (median) to adverse event sign-off
- Reduced number of monitor queries
- Increased data quality and completeness
The challenge: Cumbersome paper logs
Oncology Consultants used the industry’s standard paper logs to collect patient study data. As the established research program continued to grow, paper logs no longer fit the team’s needs, because it required the team to be in the same location to ensure timely investigator sign-off. At Oncology Consultants, Dr. Julio Peguero, Research Director, and the investigators often rotate between locations, meaning sign-off could be delayed if the investigator left the location. “This resulted in delays that were not keeping with study sponsor expectations,” explained Research Department Administrator Laura Guerra, RN, CCRC. The paper log also allowed variation in data entry, such as different date notations, that could lead to monitor queries. Updates and corrections to the log could make it difficult to read.
As a temporary solution, the team created a template in their EHR to replace the paper log. This allowed all members to work on the log regardless of location. However, it was still difficult to track the time to log review and sign-off, a key quality metric.
 Angel Leung, Flatiron and Joel Lisneros, Clinical Research Lead
Angel Leung, Flatiron and Joel Lisneros, Clinical Research Lead
The solution: Structured logs in OncoEMR®
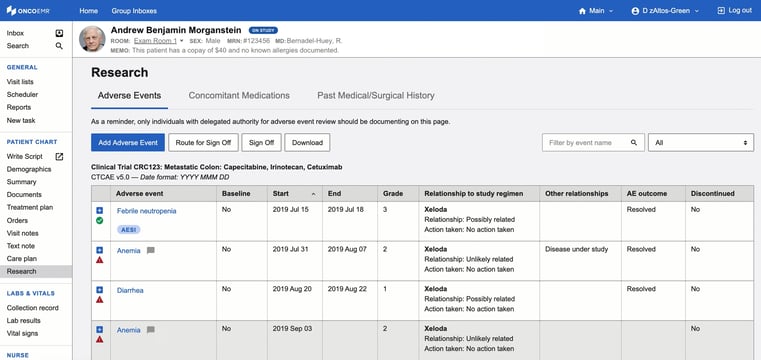
The outcome: Timely documentation and sign-off
Just a few months into using the electronic adverse events log, Oncology Consultants has already seen both improvements in time to sign off and fewer monitor queries. The median time to investigator review and sign-off is only five hours, versus the 24–48 hours it may have taken on a paper log. “With paper, it was a manual process to determine whether adverse events were being signed in a timely manner,” Camelia said. “Currently, I can easily track which adverse events are unsigned, which improves our data quality.” Laura also shared that OncoEMR® has reduced monitor queries around data entry, especially queries due to variation in logging dates.
Oncology Consultants is using the electronic research logs in OncoEMR® for studies that support CTCAE versions 4.03 and 5.0. They are also continuing to partner with Flatiron to develop additional electronic research documentation logs in OncoEMR®.
Research Quality Assurance Manager, Oncology Consultants
This allows me to easily track adverse events that are unsigned by investigators, improving data quality.