In today's rapidly evolving landscape of accelerated drug development, reliance on data derived from traditional randomized control trials (RCTs) is shifting toward a combination of RCT data and real-world data (RWD) from sources like electronic health records (EHRs), health insurance claims, social determinants of health (SDoH), and patient surveys.
A growing body of literature1 suggests RWD promises to advance our understanding of critical aspects within clinical pharmacology and translational science. Clinical pharmacology plays an important role in the drug development process to determine the optimal dosing, taking into consideration both intrinsic factors (e.g., organ impairment, genetic polymorphisms) and extrinsic factors (e.g., dietary habits, drug clearance of concurrent medications).
Including RWD in clinical pharmacology research is an exciting development because there is a serious lack of dosing information on patients often excluded from clinical trials, including protected vulnerable populations. Clinical trials typically enroll both younger and healthier patients, which may not fully represent the population for whom the drug will be indicated. Therefore, uncertainty in clinical management remains for patient populations who were not studied in clinical trials.
Using RWD for post-marketing requirements / commitments and label updates
Including information on patients with organ dysfunction improves labeling and promotes the safe and effective use of medical products across a broader patient population likely to use the drug. Health authorities like the FDA often try to correct the gaps in clinical trial data by issuing post-marketing requirements (PMR) and/or commitments (PMC) from clinical researchers to further evaluate dosing in patient populations that were not included in a clinical trial. For the 153 U.S. Food and Drug Administration (FDA) oncology drug approvals from January 1, 2021 to December 31, 2022, there were 272 clinical PMCs/PMR issued; of these, 24 were PMRs related to further evaluation of dosing in patients with kidney or liver dysfunction.
RWD collected during routine clinical care can help bridge this knowledge gap by informing benefit/risk assessments and enabling the monitoring and evaluation of dosing, compliance, and adherence — ultimately improving patient management in these subpopulations. Safety and efficacy data in these patients can be helpful in determining if dose adjustments and/or contraindications are necessary. RWD can also be leveraged to streamline the eligibility criteria of clinical trials and inform dosing for dedicated organ impairment studies (i.e., to fulfill a post-marketing requirement or commitment).
Study objectives
To put theory into practice, Flatiron Health and the FDA, collaborators since 20162, joined forces to conduct a 2023 retrospective observational study3 to evaluate the generalizability of findings from clinical trials to patients with organ dysfunction, a demographic often overlooked in traditional research settings.
Focused on patients with advanced non-small cell lung cancer (NSCLC) and moderate to severe hepatic or renal impairment, the study aimed to shed light on the association between organ function and overall survival in those treated with PD-1/PD-L1 blocking antibodies.
Study methods
The study sources included patient-level RWD from Flatiron Health and RCT data submitted to the FDA pooled from various studies.
The de-identified and abstracted EHR-derived data from Flatiron Health was curated through technology-enabled abstraction, from ~280 US cancer clinics. Data points included baseline organ function and overall survival (OS) in patients with advanced NSCLC treated with PD-1/PD-L1 blocking antibodies.
The FDA pooled clinical trial data from nine randomized clinical trials supporting approvals of four PD-1/PD-L1 blocking antibodies as single-agent therapy for NSCLC.
Study findings
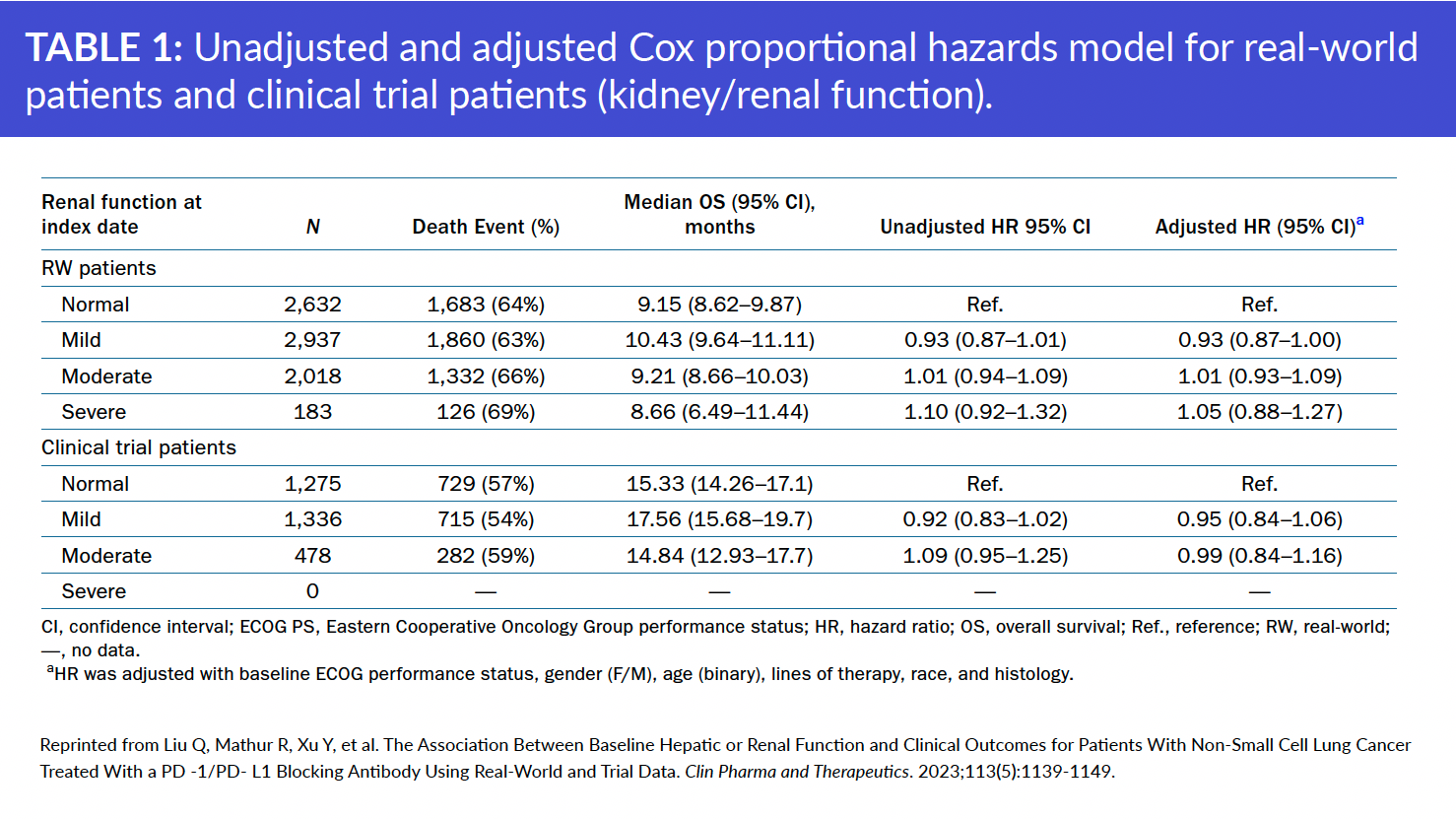
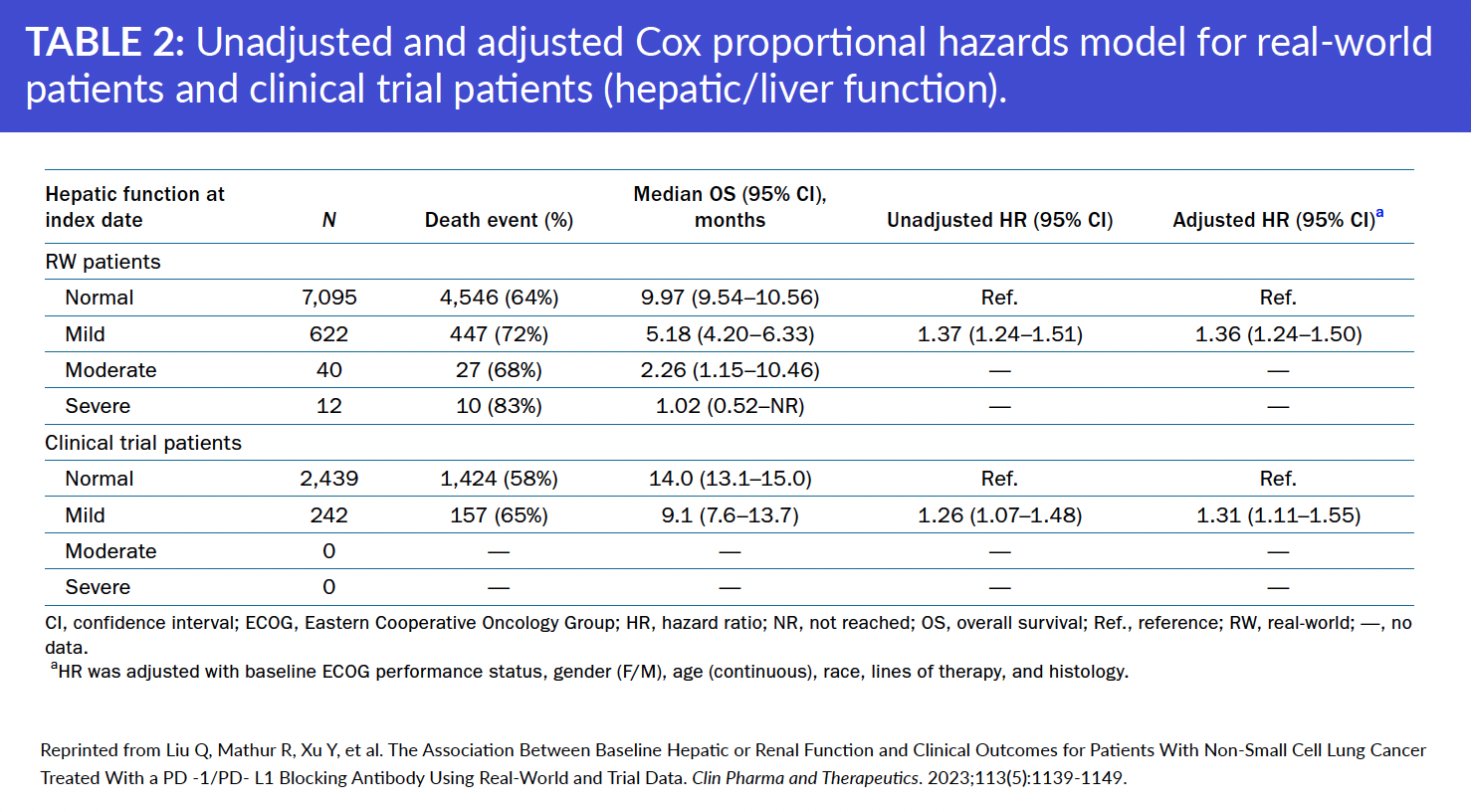
The study found that baseline kidney impairment did not appear to be associated with real-world overall survival (rwOS) in the real-world cohort or OS in the pooled clinical trial cohort. In contrast, baseline liver impairment was associated with shorter rwOS in the real-world cohort and OS in the pooled clinical trial cohort.
NSCLC patients with moderate to severe kidney or liver impairment were represented in the real-world cohort, but not in the pooled clinical trial data.
In addition, researchers found that RWD provided information on a broader range of renal and hepatic functions than was evaluated in clinical trials and improved understanding of organ-impaired populations not represented in clinical trials.
 RWD can improve generalizability of clinical trial findings
RWD can improve generalizability of clinical trial findings
Despite the limitations of the study, such as sample size, data missingness, and unmeasured confounding, this exploratory study demonstrates the potential opportunity for RWD to improve drug development for patients with organ impairment by characterizing outcomes for a subset of patients not usually included in clinical trials.
By including data from overlooked populations, using RWD to supplement RCT can improve the generalizability of study findings, fostering more equitable and personalized healthcare practices and better-informed treatments. These results may also provide evidence supporting the expansion of eligibility criteria in clinical trials. This collaborative research shows promise that RWD can complement clinical trial data to better understand populations underrepresented in clinical trials and support drug development and communications to healthcare practitioners.
References
-
Zhao X, Iqbal S, Valdes IL, Dresser M, Girish S. Integrating real‐world data to accelerate and guide drug development: A clinical pharmacology perspective. Clinical Translational Sci. 2022;15(10):2293-2302.
-
Liu Q, Mathur R, Xu Y, et al. The Association Between Baseline Hepatic or Renal Function and Clinical Outcomes for Patients With Non‐Small Cell Lung Cancer Treated With a PD ‐1/PD‐ L1 Blocking Antibody Using Real‐World and Trial Data. Clin Pharma and Therapeutics. 2023;113(5):1139-1149.
-
Flatiron Health. (2023, September 6). Flatiron Health Enters New Five-Year Research Collaboration with FDA on Real-World Data [Press release]. Retrieved from https://flatiron.com/resources/flatiron-health-enters-new-five-year-research-collaboration-with-fda-on-real-world-data.