Our summary
Oncologists encounter unique challenges in staying current with the latest therapies. Clinical decision support (CDS) tools like Flatiron Assist™ offer real-time access to evolving treatment guidelines. This study explores the prescription patterns of oncologists who utilized Flatiron Assist™ versus those who did not, following the FDA approval and CDS tool incorporation of a new treatment regimen.
Specifically, the research focuses on the ordering trends related to nivolumab and platinum-doublet chemotherapy in neoadjuvant Non-Small Cell Lung Cancer (NSCLC).
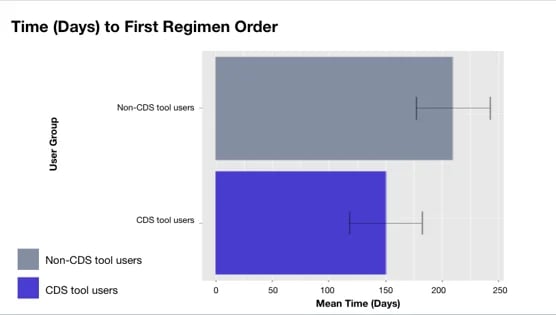 Figure. Time to first nivolumab/platinum doublet order post-incorporation in CDS tool. The FDA approved this treatment on March 4, 2022 and updates were incorporated in the CDS tool on April 12, 2022
Figure. Time to first nivolumab/platinum doublet order post-incorporation in CDS tool. The FDA approved this treatment on March 4, 2022 and updates were incorporated in the CDS tool on April 12, 2022
Why this matters
The study’s findings underscore the significant impact of CDS tools, showing that oncologists utilizing the tool initiated the newly approved treatment 59 days earlier than non-CDS tool users. This early adoption, facilitated by real-time information, has the potential to improve patient outcomes.